CINVANTI—the IV NK1 RA that improved practice efficiencies in a time and motion study1
CINVANTI 2-minute IV Push streamlined nurse and pharmacy workflow vs 30-minute infusion, saving 33 minutes per dose1
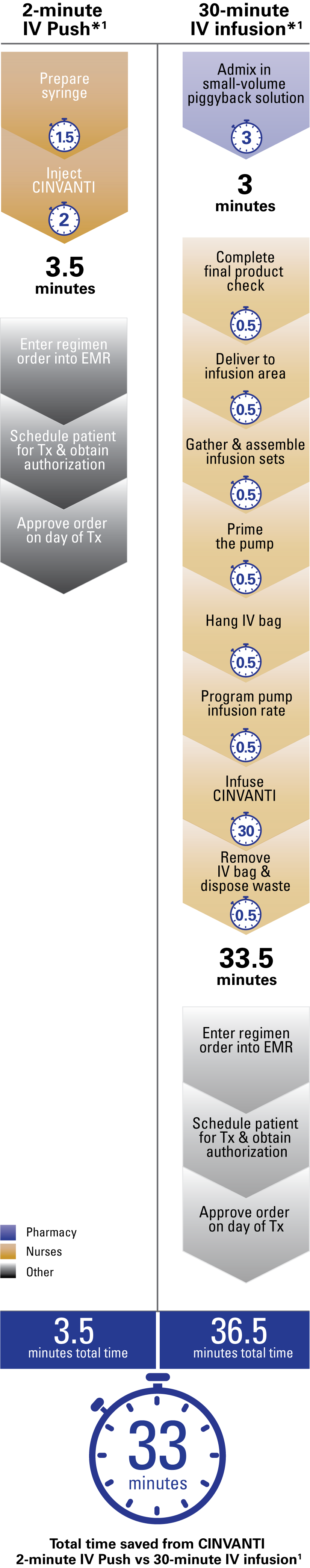
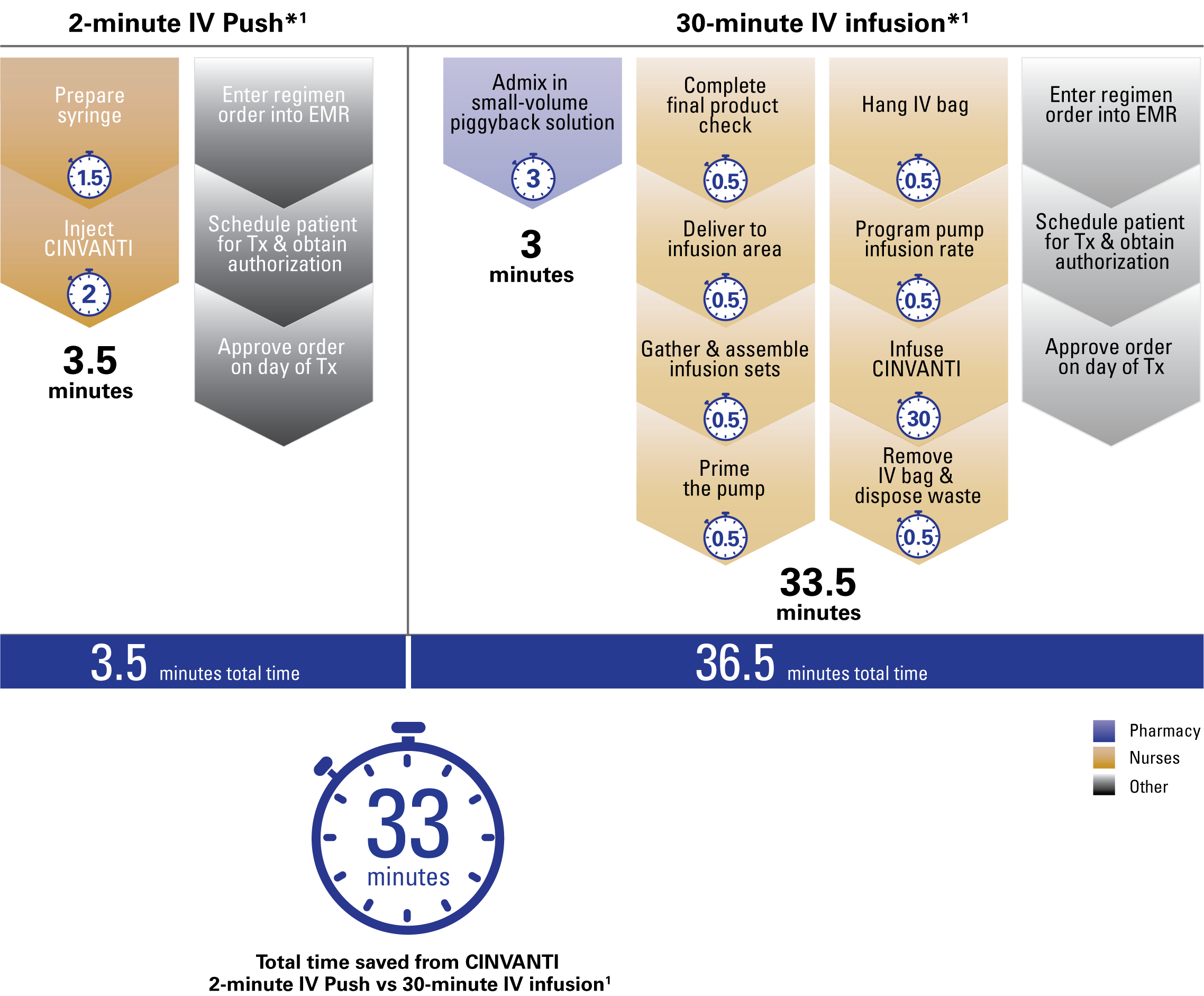
| EMR=electronic medical record; IV=intravenous; NK1 RA=neurokinin-1 receptor antagonist; Tx=treatment. | |
| * | Some common workflow steps for both methods of administration have not been included in this chart. |
CINVANTI 2-minute IV Push eliminates key steps from the preparation and administration required for IV infusions1
Time saved with CINVANTI 2-minute IV Push from steps eliminated vs IV infusions
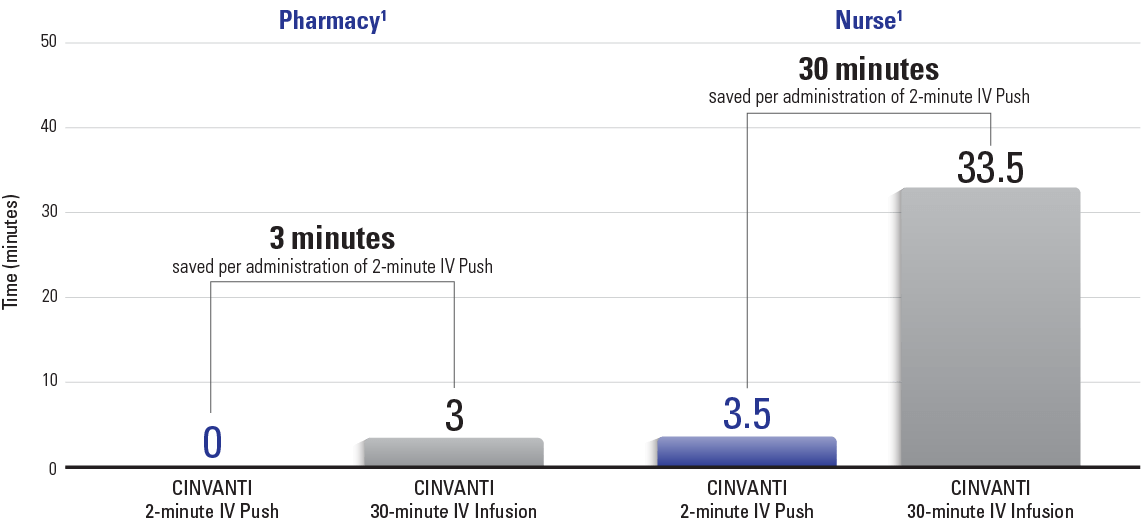
Steps removed by using CINVANTI 2-minute IV Push vs 30-minute IV infusion*1:
| Pharmacy | Nurse |
| Mixing small-volume parenteral solution | Verifying admixture is correct prior to administration |
| Delivering to the infusion area | |
| Gathering and assembling infusion sets | |
| Priming the pump/tubing | |
| Hanging the IV bag | |
| Programming the pump infusion rate | |
| Infusing the product | |
| Removing the IV bag when completed and disposing of waste appropriately |
*Observational study with inputs from nurses, pharmacy leads, and pharmacy technicians.
IV=intravenous.
CINVANTI 2-minute IV Push shortens infusion chair times1
- Allows for savings on chair time that could be reused for other procedures1
- Optimizes infusion schedules, allowing a greater number of patients to be treated in a more timely fashion1
- Can help prevent unscheduled delays due to antiemetic preparation and complicated bottlenecking, helping to avoid frustration for employees and patients1
CINVANTI 2-minute IV Push may reduce administrative work1
Nurses are able to redirect time for enhanced patient care, thoroughly reviewing chemotherapy or other orders, and assisting other nurses1
Pharmacy is able to complete other tasks such as organizing and cleaning the pharmacy, performing inventory management, drug ordering, and correcting charge documentation1
IV=intravenous.
The need for materials such as tubing and bags of parenteral solution was eliminated with CINVANTI 2-minute IV Push1*
Supply item |
Cost |
CINVANTI
|
CINVANTI
|
Single pair of gloves |
$0.16 |
 |
 |
20 mL Luer Lock plastic syringe (sterile) |
$0.27 |
 |
 |
18 g needle (sterile) |
$0.04 |
 |
 |
Alcohol swab (sterile) |
$0.02 |
 |
 |
100 mL NS infusion bag (sterile) |
$1.22 |
N/A |
 |
Secondary tubing set (sterile) |
$0.77 |
N/A |
 |
Patient label |
$0.03 |
 |
 |
Total |
$0.52 |
$2.51 |
|
|
IV=intravenous; N/A=not applicable; NS=normal saline. |
 |
||
IV=intravenous.